

These two sugars only differ by one -OH group being changed to an -H, but provides different capabilities for each molecule. On on the other hand, the sugar in the backbone of RNA is called ribose. In DNA, the sugar involved is deoxyribose. However, their sugar phosphate backbone differs slightly. In the structure below, each nucleotide is drawn in a different color. While the introduction of one or even two nicks in the sugarphosphate backbone yields no detectable effect on electron transfer, a CA mismatch significantly.
#PHOSPHATE BACKBONE FREE#
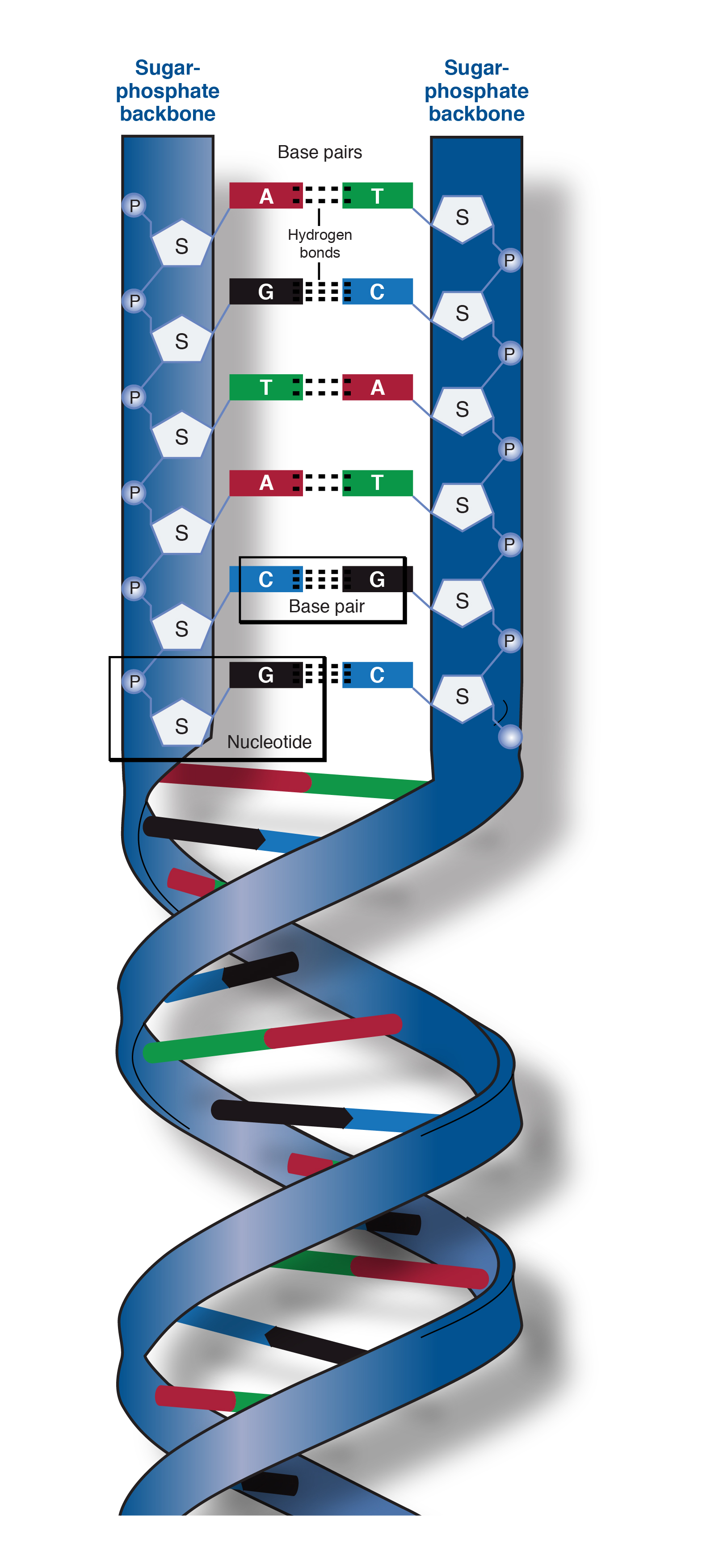
RNA and DNA are both examples of phosphodiesters and have a very similar structure. The backbone has a 5 end (with a free phosphate) and a 3 end (with a free OH group). Short Answer What is the role of phosphodiester bonds within the sugar-phosphate backbone of DNA Microbiology Chapter 10. They are extremely important in the function of DNA. These sugars are linked together by a phosphodiester bond, between carbon 4 of their chain, and a CH 2 group that is attached to a phosphate ion. It consists of 5-carbon deoxyribose sugars and phosphate groups. One turn of this helix is 34nm long, the diameter of it is 2nm, and there are ten bases attached per turn at 0.34nm. The sugar phosphate backbone is an important stuctural component of DNA. These features make DNA can repel water and would not hydrolysed and breakdown by the aqueous environment. DNA is very stable due to rungs of “ladder” is hydrophobic and phosphate sugar backbone of DNA is negatively charged. The purpose of this twisting is to protect the bases inside it, and prevent them from being damaged by the environment. one runs 3' to 5', the other run 5' to 3'. This is done by the sugar phosphate backbone twisting around itself in a coil. School of Chemistry and Biochemistry, Georgia Institute of Technology, Atlanta, Georgia 30332-0400, USA.Figure 1 Diagram showing the sugar phosphate backbone of DNA, and the nitrogenous bases attached to it, forming a nucleotide Structure of DNAĭNA is wound into an right-handed double helix. This bending may be related to the direct coordination of a sodium cation by a DNA base, with unprecedented inner-shell (direct) coordination of penta-hydrated sodium at the O6 atom of a guanine. Electrostatic forces appear to induce modest DNA bending into the major groove. TriplatinNC extends along the phosphate backbone, in a mode of binding we call "Backbone Tracking" and spans the minor groove in a mode of binding we call "Groove Spanning". The high repetition and geometric regularity of the motif suggests that this type of Pt(II) center can be developed as a modular nucleic acid binding device with general utility. The interaction appears to prefer O2P over O1P atoms (frequency of interaction is O2P > O1P, base and sugar oxygens > N). The geometry is conserved among the 8 observed phosphate clamps in this structure. The three square-planar tetra-am(m)ine Pt(II) coordination units form bidentate N.O.N complexes with OP atoms, in a motif we call the Phosphate Clamp. The helix is right-handed with 10 bp per turn. Frank-Kamenetskii, in Encyclopedia of Condensed Matter Physics, 2005 B-DNA It (see Figure 4a) consists of two helically twisted sugar-phosphate backbones stuffed with base pairs of two types, AT and GC. Instead, it binds to phosphate oxygen atoms and thus associates with the backbone. Phosphate Backbone DNA and RNA, Biophysical Aspects M.D. The pairing of the nitrogenous bases that are connected to the sugar-phosphate backbone play a key role in the ability of DNA to store and transfer genetic information. The structure of DNA is tied to its function. TriplatinNC does not intercalate nor does it bind in either groove. The sugar-phosphate backbone, as mentioned, is an important component of DNA's double helix structure. The classic double-helical structure of B-DNA, proposed by Watson and Crick1, is governed by hydrogen bonds. TriplatinNC is a multifunctional DNA ligand, with three cationic Pt(II) centers, and directional hydrogen bonding functionalities, linked by flexible hydrophobic segments, but without the potential for covalent interaction. ing interaction, sugar-phosphate backbone. We describe a 1.2 A X-ray structure of a double-stranded B-DNA dodecamer (the Dickerson Dodecamer, DDD, 2) associated with a cytotoxic platinum(II) complex, (TriplatinNC). The sugar-phosphate backbone forms the structural framework of nucleic acids, like DNA and RNA, and is composed of alternating sugar and phosphate groups.


 0 kommentar(er)
0 kommentar(er)
